Researching for seasonal synergies: Powering hydrogen peroxide production with Europe’s wind and sun
Europe’s abundant wind and solar power offer a unique chance to produce hydrogen peroxide sustainably and locally. Power2Hype develops electrochemical technology that converts surplus renewable electricity into this essential chemical, matching seasonal energy availability with industrial demand. This flexible approach supports clean energy storage and cuts carbon emissions in chemical production.
Europe’s shift to clean electricity is also opening doors for climate-friendly chemicals like hydrogen peroxide (H₂O₂). Traditionally made in large plants via energy-intensive chemistry, H₂O₂ could increasingly be produced on-site with electricity from wind and solar farms. In the power2hype production scenario, excess renewable power is turned into a useful chemical, storing clean energy in liquid form. This idea is gaining traction because Europe’s renewable supply naturally cycles through the year: wind farms surge in winter while solar peaks in summer. Such seasonality can be matched with H₂O₂ demand, creating a new synergy between green power and green chemistry.
European wind and solar output vary by season, this is already significantly affecting the power systems and prices in Europe. You can see the example of a fever curve-like production and price profile for the past month of April in the figures below.
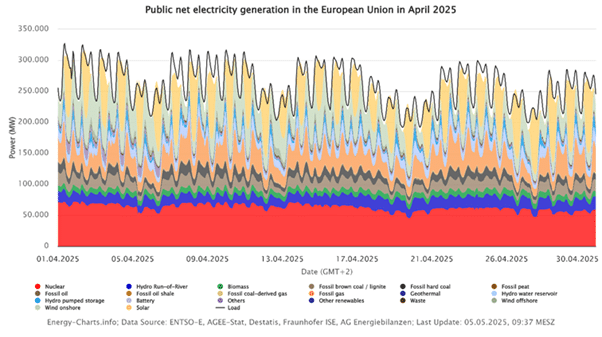
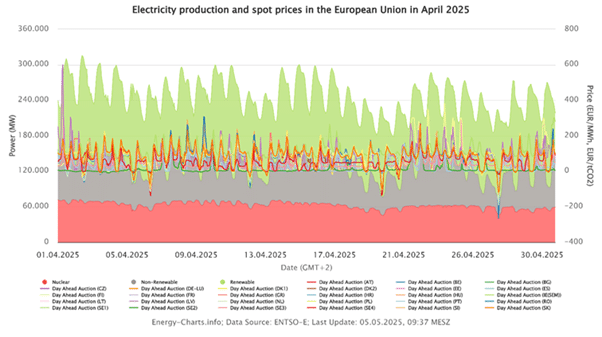
Wind turbines tend to produce the most power in winter nights, when solar panels yield little. In contrast, long summer days drive high photovoltaic generation. This complementary pattern means there are seasonal “shoulders” of surplus renewable electricity that could feed small electrochemical plants. By running H₂O₂ electrolyzers during these surpluses, regions could capture wasted green energy and make it into a storable, on-demand chemical.
To see how this could play out, consider real-world examples. In summer, solar farms in Southern Europe often generate more power than local demand. At the same time many industries ramp up work. For instance, pulp and paper mills typically operate at high capacity to meet seasonal demand, and they use large amounts of H₂O₂ for bleaching. An on-site electrolyzer coupled to a solar plant could produce the needed peroxide right when the mill is running full tilt. Likewise, water treatment and chemical plants that use H₂O₂ for disinfection or oxidation could schedule their peroxide production for sunny or windy periods. For example, advanced oxidation processes (which combine H₂O₂ with UV light or catalysts to break down pollutants) are often needed in summer to combat algae or industrial wastewater bursts. By timing electrochemical H₂O₂ generation to coincide with these peaks, industries can be partly self-sufficient in peroxide supply.
Such examples show that seasonal high points in renewable electricity can align with real chemical needs. Instead of curtailing excess power or exporting it at low prices, operators could flexibly run electrolyzers to make H₂O₂, storing the results for when it’s needed. This not only balances the grid, but also cuts CO₂ by avoiding petrochemical H₂O₂.
Scaling up the synergy
Turning this vision into reality will require concerted effort. Key steps include:
- Advanced catalysts and cell designs: To be competitive, electrochemical H₂O₂ production must improve. The power2hype researchers are developing catalysts that boost yield and selectivity of the 2‑electron ORR, and electrolyzer designs (e.g. gas-diffusion electrodes) that can handle higher currents. Better materials could lower the electricity needed per kilogram of H₂O₂, and allow compact units.
- Smart controls and demand forecasting: Modern energy management systems can link renewables to chemical plants. For example, a smart controller could monitor grid conditions and automatically start the H₂O₂ electrolyzer when wind output spikes or solar midday peaks. Forecasting weather and renewable output also helps schedule production. In effect, H₂O₂ plants would become “virtual batteries”, running only on excess power.
- Storage and distribution infrastructure: Although H₂O₂ is safer than many chemicals, it still requires care. We need buffer tanks and pipelines to store generated H₂O₂ until use. Modular storage systems could allow factories to stock up in advance. In some cases, peroxide could even be converted into solid carriers (like urea-H₂O₂ adducts) for easier handling.
- Regional coordination: Because wind patterns and industrial demand differ across Europe, sharing resources will help. Windy Northern countries could export surplus power or peroxide to others, while sunny South sends excess solar. Cross-border collaboration and grids can smooth out local shortfalls.
In the end, electrochemical H₂O₂ offers a promising match for Europe’s renewable rhythms. By making chemicals when the wind blows hardest and the sun shines brightest, we create a flexible, low-carbon production network. With further innovation and smart planning, turning seasonal surpluses into useful chemicals like hydrogen peroxide could become a common practice – a win for grids, industries, and the climate.


